CORRECTION OF OF THIS POST
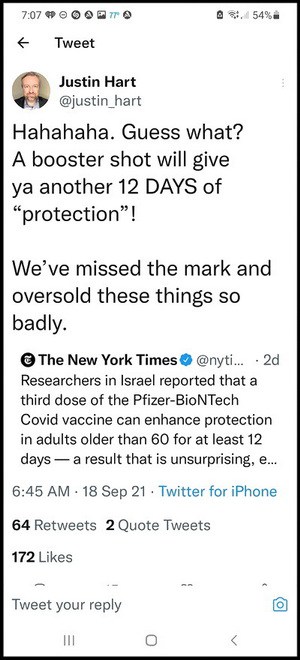
A Facebook comrade (M.B.) pointed out that the NEW YORK TIMES TWITTER graphic is wrong. Either by a computer generated error or human error. What is not pointed out CLEARLY, which I see now, is that in the twelve days studied there was a benefit, and common sense would say there would be after that 12-days. But that is the only time span studied.
Some interpreted it thus:
Again, I agree with M.B., this is taken from it’s context.
The SEATTLE TIMES didn’t make this too clear either:
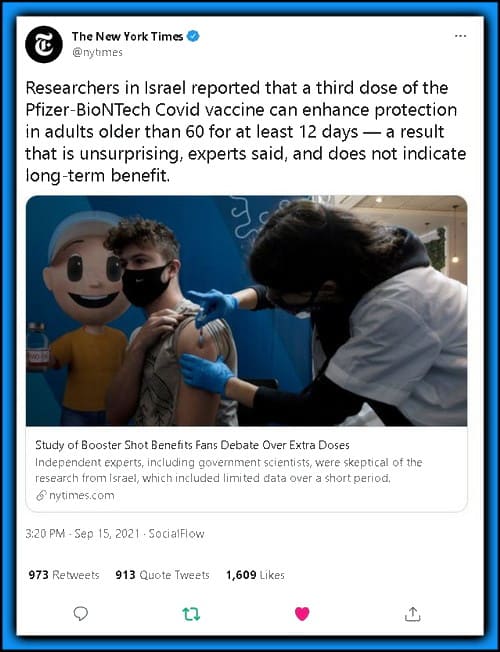
- Wading into an acrimonious debate over booster doses, researchers in Israel reported Wednesday that a third dose of the Pfizer coronavirus vaccine can prevent both infections and severe illness in adults older than 60 for at least 12 days.
I can see how that is misinterpreted, which I was a party to as well. Here is — for instance — the NYT’s quote that seems to prove M.B.’s point:
….In the new study, the Israeli team collected data on the effect of booster shots, based on the health records of more than 1.1 million people over age 60. At least 12 days after the booster, rates of infection were elevenfold lower and of severe disease nearly twentyfold lower in those who received a booster compared with those who had received only two doses, the researchers found.
The researchers acknowledged that their results were preliminary. “We cannot tell at this point what will happen in the long run,” said Micha Mandel, a professor of statistics and data science at the Hebrew University of Jerusalem…..
YAHOO NEWS notes the same:
….The main finding was the older population [60+], when boosted, was 11 times less likely to get infected and 19.5 times less likely to get severely ill compared to similar people who had received two doses but not a booster shot.
[….]
In the Israeli study, the group that didn’t get boosters recorded 4,439 infections and 294 severe illnesses. The booster group had 934 infections and 29 severe cases. The risk reduction rates accounted for the fact that the two groups were not even in size, as far more people joined the booster group over time.
[….]
Additionally, the study has a very limited follow-up time, and doesn’t show how long protection from boosters may last. That’s an essential question in figuring our whether a booster campaign is worth launching.
The study’s limited duration may skew its findings. Researchers started counting cases for the booster group only when they are 12 days removed from the third dose.
It can take up to a month on average for a person to go from exposed to infected to seriously ill, Murray said. Therefore, the study may not include enough follow-up time to show the true effect of the boosters.
There aren’t any high-quality studies on booster shots “It’s not clear to me that there’s anywhere near enough follow-up time, even for the earliest boosters,” Murray said.
“All of these problems together make it really hard to know how much we can trust that number that comes out of the study,” Murray added…..